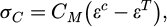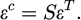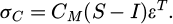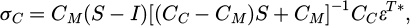| Issue |
Mechanics & Industry
Volume 25, 2024
|
|
|---|---|---|
| Article Number | 12 | |
| Number of page(s) | 12 | |
| DOI | https://doi.org/10.1051/meca/2024008 | |
| Published online | 09 April 2024 | |
Original Article
Optimization of large cast Haynes 282 based on thermal induced cracks: formation and elimination
Department of Transport Engineering, Nanjing Vocational University of Industry Technology, Nanjing, Jiangsu, China
* e-mail: yujin.yang@niit.edu.cn
Received:
4
April
2023
Accepted:
1
March
2024
Haynes 282, a wrought material, attracts the attention from casting application due to its excellent mechanical properties. Fracture of large cast Haynes 282 were found before yielding during tensile test in previous researches, but the reasons have not been fully understood. In this study, various heat treatments were designed and applied for large cast Haynes 282 to reveal the fracture before yielding. Thermodynamic calculation, residual stress calculation combined with microstructural examination were utilized to analyze the reasons of fracture. It has been found that there are grain boundary cracking and grain boundary precipitate cracking in the tensile sample. The grain boundary cracking is mostly attributed to the melting of the low melting point phases such as some segregated matrix and μ phases. The grain boundary precipitate cracking is associated with the blocky Ti, Mo rich MC carbides, and it is more likely to be caused by the quenching after the second homogenization treatment. Optimized heat treatment has been proposed and proven to be workable for the large cast Haynes 282.
Key words: Large cast Haynes 282 / microstructural characterization / crack mechanism / properties optimization
© Y. Yang, Published by EDP Sciences, 2024
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1 Introduction
Increasing working temperature and pressure of power plant is an important way to reduce environmental impact of power generation industry [1]. However, the key challenge is to select or develop suitable materials to withstand increasingly aggressive environment. Haynes 282 (H282) is one of the candidate materials [2]. Although it was firstly developed as a wrought materials in 2005 [3], due to its good creep strength, weldability and fabricability [4,5], it attracts the attention from casting applications.
Previous researches have studied cast H282 with various sizes [6–8], it can be concluded that reduction in tensile strength of cast H282 is a tradeoff for the increased cast size (Tab. 1). Comparing with wrought H282, nearly 50% reduction in tensile strength was found in a 170 kg cast H282, moreover, fracture before yielding is found in a large (1500 kg) cast H282. The reduction of tensile strength of cast H282 is attributed to the microcracks generated during casting in the inter-dendritic region [7], however, the formation of microcracks has not been addressed, the fracture before yielding of the large cast H282 is not explained [8]. It is necessary to investigate the relevance of microcracks, microstructure and heat treatment.
Heat treatment and microstructure are of crucial importance to tensile strength [9,10]. For a similar sizes H282 made by additive manufacture (AM) treated by different heat treatments, the tensile strength of some AM H282 is only slighter lower than that of the wrought H282 [11–13], however, that of some AM H282 is in the same level of the cast H282 [14]. The microstructure of H282 has been detailed studied [6,15–18], which includes MX, Cr rich M23C6, Mo rich M6C and uniformly distributed γ/ [15]. Some other phases were particularly found in some special cases, such as borides in in welded H282 [16], eutectic phases in a thin-walled cast component [6], and grain boundary Mo rich μ phases in large cast [18]. It has been reported that the dissolution of the carbon-boride during heat treatment can cause HAZ cracking in wrought H282 [19], while whether this is the case for cast H282, the reason of microcracks in cast H282 have not been reported, although this knowledge is necessary for cast H282 application in power generation industry such as casing and valves. The aim of the present study is to fill this gap. Additionally, the formation mechanism of two types of microcracks has been elaborated theoretically and experimentally.
In this work, the fractured samples were examined in different locations to determine the types of cracks. A series of breakdown heat treatment were designed to illustrate the effect of different stages of heat treatment on microstructure and cracks, and furthermore to localize the formation of the cracks. By microstructural examination, 3-D characterization technique, thermodynamic and stress calculation, the formation mechanism of cracks found in fractured tensile sample were addressed. Base on the findings, optimization of heat treatment to eliminate the cracks has been proposed and applied, the microstructure and tensile results of the H282 after optimized heat treatment shows that the optimization works for the large cast H282.
Room temperature tensile properties of both wrought, AM and cast H282.
2 Materials and methods
2.1 Materials and heat treatments
The materials studied in this study were manufactured using traditional sand casting by Goodwin Steel Casting (GSC) LTD. The weight of a cast step block was in the order of 1500 kg and the examined samples were cut from middle of the thin and thick sections as shown in Figure 1. The nominal chemical compositions of the cast are illustrated in Table 2.
Samples were heat treated using both chamber furnaces and an 805A/D dilatometer. Silica rods were used to hold the samples and the heating curves recorded were used to calculate the coefficient of thermal expansion (CTE) of the alloy. For microstructural examination, all samples were mounted in electrically conductive Bakelite and ground on SiC papers from 220 to 1200 grit and then polished with a series of diamond solutions to a 1 μm finish. A detailed microstructural study was carried out by using a Carl Zeiss (Leo) 1530 VP field emission gun scanning electron microscope (FEG-SEM) equipped with Oxford Instruments energy-dispersive X-ray spectrometers (EDS). A 3-D reconstruction was made by reconstructing focused ion beam (FIB) slice and view images using Avizo software.
Room temperature tensile tests were conducted with a constant crosshead speed of 1.5 mm/min. on specimens underwent the pre-service heat treatment (HT1) [8] which includes a two-step homogenization and a two-stage ageing treatment as advised from Jablonski et al. [20], to reappear the phenomenon of the facture before yielding of the large cast. To reveal the relationship of failure, microstructure and heat treatment, samples cut from the thick section were undergone a series of heat treatments as illustrated in Table 3: a one-stage homogenization (HT1-1), a two-stage homogenization (HT1-2), and the two-stage homogenization plus the first ageing (HT1-3), and the whole HT1 heat treatment. To eliminate cracks, an optimized heat treatment (HT2) was applied, which includes a homogenization at 1100 °C for 12 h followed by a ramp up to 1150 °C, holding for 15 h followed by water quenching, then first aged at 1010 °C for 5 h followed by water quenching, finally second aged at 788 °C for 15 h followed by air cooling. Specimens cut form the thin and thick sections after the HT2 underwent room temperature tensile test with a constant crosshead speed of 1.5 mm/min.
 |
Fig. 1 A picture of a cast step block (thin section, thick section) of the cast H282 materials; red dash lines are cutting lines. |
The nominal composition in wt. % of H282.
Heat treatment of all samples in this study.
2.2 Modelling procedure
2.2.1 Thermodynamic calculation
Thermodynamic calculations were conducted with a commercial software package, MTDATA, developed by the National Physical Laboratory [21] and an appropriate Ni-database developed between Thermotech Ltd and Rolls-Royce Plc [22]. Thermodynamic equilibrium calculations were utilized to calculate the equilibrium pseudo phase diagram according to the quantified chemical compositions.
2.2.2 Interface stress calculation
Interface carbides cracking susceptibility was calculated in this study. Assume carbide as an inclusion in matrix, the interface can be treated as a “metal-ceramic-metal” bonding system. In this system, depending on the magnitude and sign of the residual stress, cracks caused by residual stress build up can happen in the ceramic or at the interface between the ceramic/metal bonds. When the ceramic/metal systems are subject to a positive misfit (αmetal > αceramic), cracks are found in the ceramic, and when the systems are subject to a negative misfit (αmetal < αceramic), cracks are found along the interface [23]. Where αmetal and αceramic are values of the coefficient of thermal expansion (CTE) of metal and ceramic respectively.
The stress within the carbides (σC) can be calculated using Hooke's Law in terms of the elastic strain (εc-εT) and the stiffness tensor of the matrix (CM) as shown in equation (1)
where εT is a specified shape change, εc is the final constrained strain, and it can be obtained from εT by means of a tensor which is termed the Eshelby S tensor (Eq. (2)). Eshelby S tensor can be calculated in terms of the aspect ratio of the carbide and the Poisson's ratio of the matrix [24].
Therefore, equation (1) can be changed to equation (3):
Considering the differential thermal contraction misfit as a shape change (ϵT* = (αM − αC)ΔT) [24], the stress within the carbides (σC) in terms of its stiffness (CC) and the elastic strain can be written as equation (4):
From equations (3) and (4), the transformation strain εT can be deduced for any shape change and stiffness mismatch (CC-CM) between the two phases. The carbide stress is thus can be written as equation (5):
where εT* is the stress-free transformation strain, CM, CC, S and I are respectively the stiffness tensors of matrix and the carbide, Eshelby tensor and identity tensors, and αM,αC and ΔT are respectively the CTE of matrix, CTE of inclusion and a temperature change. The Eshelby tensor is a function of the aspect ratio of the carbide (s) and the Poisson's ratio of matrix (ν).
3 Results and discussion
3.1 Microstructure of tensile sample underwent HT1
The large cast H282 underwent HT1 fractured before yielding and the micrographs are presented in Figure 2. Both grain boundary cracks and grain boundary precipitate cracks (blocky Ti, Mo-rich MC carbides identified in [17]) were found in the cross section and the head parts, and this could be the reasons of the low-ductility failure of the large cast H282. The formation mechanisms of these cracks are explained in Sections 3.3 and 3.4 respectively. In the tensile head sample, cracks in grain boundary precipitates are formed along the grain boundary, indicating that these cracks are formed after HT1 before tensile test.
 |
Fig. 2 Micrographs of H282 after the HT1(tensile sample): grain boundary cracks and grain boundary precipitate cracks. |
3.2 The effect of heat treatments grain boundary MC
To address when and why these cracks are formed, the HT1 treatment was broken down into four stages to study its effect as follows:
HT1-1: Homogenization at 1100 °C for 6 h followed by water quenching;
HT1-2: Homogenization at 1100 °C for 6 h and then holding at 1200 °C for 12 h followed by water quenching;
HT1-3: Homogenization at 1100 °C for 6 h and then holding at 1200 °C for 12 h followed by water quenching, and then first aged at 1010 °C for 5 h followed by water quenching;
HT1-4: The whole HT1 treatment (equals the HT1 treatment).
Micrographs of grain boundaries which are decorated by the blocky Ti, Mo-rich MC carbides after the HT1-1, HT1-2 and HT1-3 and HT1-4 are presented in Figures 3a, 3b, 3c, and 3d respectively. After holding at 1100 °C for 6 h, the grain boundaries are still covered by small white precipitates and large blocky Ti, Mo-rich MC carbides (Fig. 3a), indicating that their solvus temperature are above 1100 °C. The small white precipitates in Figure 3a could be Mo rich M6C or Mo rich μ phase which have been identified in [18]. After the HT1-2 (Fig. 3b), the small white precipitates have been dissolved since the holding temperature (1200 °C) is higher than its solvus temperature. However, the Ti, Mo-rich MC carbides are still presented due to their high solvus temperature. Comparing Figures 3a and 3b, it can be found that the blocky Ti, Mo rich MC carbide is only cracked after the HT1-2 treatment. After the HT1-3 (Fig. 3c), the blocky Ti, Mo rich MC carbide is still cracked and the Mo rich M6C phases precipitate out on and near grain boundaries. This is because that the holding temperature (1010 °C) is in the temperature window of the M6C stability range (790–1095 °C) [18]. After the HT1-4 (Fig. 3d), the Ti, Mo-rich MC carbide is still cracked, in addition, there are small homogeneously distributed γ/ particles in addition to the microstructural features after the HT1-3. Therefore, it can be concluded that it is the 1200 °C homogenization treatment leads to the cracks.
 |
Fig. 3 The effect of the HT1 on grain boundary blocky MC: (a) after the HT1-1 treatment; (b) after the HT1-2 treatment; (c) after the HT1-3 treatment and (d) after the HT1-4 treatment. |
3.3 Formation of grain boundary cracking
Seen from Figure 2, the grain boundary cracks seem to be caused by localized melting of chemically segregated regions which could be segregated matrix and/or some secondary phases. During casting, there is a large degree of segregation during solidification, the composition of the liquid is different at different undercoolings, and consequently accounts for a gradient in composition within the solid. The compositional difference will result in different melting points in different regions. As stated in previous work, the temperature of the last liquid of H282 to solidify is about 1120 °C [18], which is far lower than the homogenization temperature of 1200 °C. Therefore, it is possible that grain boundary cracks (Fig. 2) are caused by localized liquidation/incipient melting due to too high homogenization temperature.
At the same time, it is also possible that some specific grain boundary phases were melted during the homogenization treatment. Different decorating precipitates have been found in previous work [18] including blocky MC, M23C6, M6C and μ phase. Among them, the blocky MC phase has the highest solvus temperature and therefore, it is unlikely to change because of the homogenization treatment. This can also be confirmed from Figure 3 where blocky MC phases are found on grain boundaries after homogenization treatment. The other three phases (M23C6, M6C and μ phase) all have the potential to be melted during homogenization.
After homogenization at 1100 °C, M23C6 is no longer stable, whereas some grain boundaries are still covered by the M6C or μ phase (Fig. 3a). After homogenization at 1200 °C, these M6C or μ phase are no longer stable (Fig. 3b). Therefore, the grain boundary cracking could be related to be melting of either the M6C or the μ phase. The chemical composition of both the μ phase and M6C in the as cast condition were measured and illustrated in Table 4. Equilibrium phase diagrams of M6C and μ phase have been calculated using the measured chemical compositions (Tab. 4) and the results are presented in Figures 4a and 4b respectively.
In Figure 4a, there are no liquid phases found at 1200 °C and this indicates that during homogenization at 1200 °C, grain boundary cracking is not caused by the melting of M6C phase. However, in Figure 4b, there is about 50% liquid and 50% μ phase at 1200 °C, and this means that during holding at 1200 °C, μ phase is possible in liquid state. This is indirectly confirmed in the weld H282 [19]. Singh et al. [19] found that grain boundary cracking is caused by dissolution of B rich phases due to too high homogenization temperature (1190 °C). Since Mo has high potential to be combined with B, the melted precipitates are possible to be Mo rich, therefore, the grain boundary cracks could be caused by the melting of Mo rich phase in H282, and it is Mo rich μ phase to be melted in large cast H282.
 |
Fig. 4 (a) Predicted equilibrium phase diagram using the M6C composition and (b) Predicted equilibrium phase diagram using the μ phase composition. |
Quantified compositions of different Mo rich grain boundary phases in wt.% in as cast condition.
3.4 Formation of cracks in grain boundary MC
In Figure 3, cracks in the blocky carbides are propagated in the direction of the grain boundary. Since the samples are not in service or under loading, there is no extra applied stress, therefore the cracks in carbides are potentially caused by the thermal stress which was induced during cooling or heating due to thermal expansion misfit. The reason and mechanism of the carbide cracking are renationalized as follows.
Assuming the areas include the blocky carbide and matrix in Figure 3a as a metal (matrix)-ceramic (carbide)-metal (matrix) system (Fig. 5a), cracks caused by residual stress build up can happen in the ceramic or at the interface between the ceramic/metal bonds depending on the magnitude and sign of the residual stress. Cracks formed in the ceramic in a positive misfit condition, while in a negative misfit condition, it formed along the interface [23]. Therefore, the formation region of cracks is determined by the CTE of Ti, Mo-rich MC carbide and the H282 matrix.
The CTE of H282 was determined from a dilatometer heating curve (Fig. 5b). In Figure 5b, the CTE of H282 matrix at high temperature (>800 °C) agrees with the published data [3]. Since the small amount of Ti, Mo-rich MC carbide in H282, it is difficult to detect its CTE by X-ray diffraction. Values for CTE of carbides were therefore taken from literatures. Given the nature of the ‘ceramic’ bonds, the room temperature CTE of carbides is not likely to be much difference in the CTE at high temperature, therefore the room temperature of CTE of carbides were used. It has been found that the room temperature CTEs of TiC and Mo2C are 7.95 × 10−6, and 7.33 × 10−6 with a unit of °C−1 respectively [25], therefore, the CTE of Ti, Mo-rich MC carbide is likely to be between 7.33 and 7.95 × 10−6°C−1.
Comparing the CTE of H282 to the CTE of Ti, Mo-rich MC, it can be found that the CTE value of H282 is about twice of that of the Ti, Mo rich carbides, therefore, the metal (matrix)-ceramic (carbide)-metal (matrix) system is in a positive misfit condition and cracks caused by residual stress are likely to be found in the ‘ceramic’ part which is the grain boundary Ti, Mo-rich MC.
To further explain how the cracks were formed in grain boundary Ti, Mo-rich MC, the thermal misfit stress formed in carbides should be estimated. There are three stages which can introduce the thermal misfit stress: solidification after casting, heating during heat treatment and subsequent cooling during the heat treatment. Since cracks were found during heat treatment, therefore, the thermal misfit stress in carbides during heating and cooling were estimated using equation (5). The Eshelby tensor which is a function of the aspect ratio of the carbide (s) and the Poisson's ratio of matrix (ν) should be determined.
A 3-D reconstruction of a grain boundary Ti, Mo-rich MC carbide was conducted and reconstructed cracks in a Ti, Mo-rich MC carbide is presented in Figure 6. From Figures 3 and 7, the Ti, Mo-rich MC carbide can be assumed to be a prolate spheroid, where c > a = b, and the aspect ratio s = c/a (Fig. 6a). Therefore, the Eshelby S tensor of the carbide can be expressed in equation (6):
where 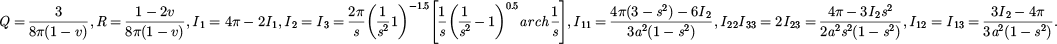
The stiffness tensor of MC (CC) was chosen as 500 GPa [25], the stiffness tensor of γ matrix (CM) was chosen as 200 GPa [3], the CTE of MC (αC) was chosen as 7.95 × 10−6 ° C−1, the CTE of γ matrix (αM) was chosen as 16.6×10−6 °C−1, and the Poisson's ratio of H282 was chosen as 0.32 [3]. The other two values in equation (5): S tensor and ΔT respectively depend on the aspect ratio of the MC and the heating or cooling rates.
To calculate the thermal misfit stress, the sensitivity of these two values were studied. Figure 7 illustrates the relationship between the thermal misfit stress σC and the aspect ratio (s) at different rates of temperature change (ΔT). It can be found that aspect ratios between 1 and 5 are sensitive to the thermal misfit stress, however, when the aspect ratio is higher than 10, the stresses tend to become independent of the aspect ratio. From Figure 7, it can be noted that the rate of temperature change has a dominant effect on the thermal misfit stress. For instance, the residual stress at a temperature rate of 100 °C/s is 10 times the thermal misfit stress at the temperature rate of 10 °C/s. The heating rate used in the heat treatment in this study was 1 °C/s, and therefore the tensile stress produced in MC during heating is about 43 MPa, which is lower than the tensile strength of the MC (65 MPa [25]). Therefore, this indicates that the carbide cracks are probably formed during cooling rather than the heating. Water quenching was used in this work after homogenization. It can be determined from Figure 7 that a 100 °C/s cooling rate can produce about 430 MPa compressive stress in the MC carbides, indicating that the compressive stress induced during cooling can higher than the compressive strength of the MC carbide (1380 MPa [25]), when the cooling rate is above 350 °C/s. However, in reality, a cooling rate of 350 °C/s can be achieved during water quenching after homogenization at 1200 °C [26]. Therefore, it is the high cooling rate which most likely causes the cracks in the grain boundary blocky MC carbides. To eliminate the carbide cracking, a slower cooling rate during heat treatment could be considered.
 |
Fig. 5 (a) A schematic diagram of the matrix-carbide-matrix system and (b) CTE plot comparing the measured and published values of H282 [3]. |
 |
Fig. 6 A 3-D reconstruction of grain boundary blocky MC with cracks. |
 |
Fig. 7 A plot of residual stress against aspect ratio as a function of different rates of change in temperature. |
3.5 Heat treatment optimization
Samples underwent homogenization heat treatment with furnace cooling were examined and the micrographs of grain boundaries were presented in Figure 8. Figures 8a and 8b is the micrograph of the sample underwent HT1-2(F), grain boundary MC is not cracked in this condition. However, due to the low cooling rate after homogenization, γ/ particles found in HT1(F) samples (about 67 nm in Fig. 8c) are larger than that in the HT1 sample (about 50 nm in Fig. 3d), this will in turn reduce the creep properties of the materials during service [27].
Therefore, to explore the possibility of a heat treatment modification for H282, only an adjustment to the heat treatment temperature has been considered. From consideration of the thermodynamic predictions [18], 1150 °C was chosen as the secondary homogenization temperature because at this temperature, nearly all secondary precipitates are predicted to be dissolved except for the MX. The first stage homogenization temperature was kept as 1100 °C, since this temperature did not cause cracks as shown in Figure 3a. In terms of holding time, a longer holding time at the first stage was chosen to provide a more homogenized microstructure prior to the secondary stage homogenization. Therefore, the HT2 was modified to be 1100 °C/12 h + 1150 °C/15 h/water quenching, and the ageing treatment was the same as the HT1. The microstructure after the HT2 of the thin and thick sections are presented in Figure 9. After the HT2, the thin section is free of cracks, however, in the thick section, localized melting is still found in few amounts, which means that 1150 °C is still too high for few heavy segregated regions in the thick section.
Table 5 illustrates the tensile properties of H282 after the HT2. The thin section has a higher yield strength and nearly three times the ductility of the thick section due to the non-existence of microstructural cracks. However, the ductility of the thick section is limited by few grain boundary cracks found in Figure 9d.
To further eliminate the few grain boundary cracks (Fig. 9d), the HT3 was adjusted to only at 1100 °C for even longer time (100 h). After underwent HT3, a few localized pores which is much smaller than that in Figure 9d, were still found in a few regions of the thick section as presented in Figure 10. However, a more than 100 hours heat treatment is not cost-effective for industries, therefore, to fully eliminate cracks in the heavy section for the large cast H282, compositional optimization should be considered. Since the grain boundary cracks was mostly caused by melting of segregated matrix and/or Mo rich μ phase, chemical optimization to reduce segregation and/or the formation of μ phase requires further investigation.
 |
Fig. 8 Micrographs showing microstructure after the HT with furnace cooling: (a, b) samples underwent HT1-2(F); (c) samples underwent HT1(F). |
 |
Fig. 9 Micrographs showing microstructure after the HT2: (a, b) the thin section; (c, d) the thick section. |
Tensile properties of thin and thick section of H282 after modified heat treatment.
 |
Fig. 10 A micrograph of thick section after HT3 (homogenized at 1100 °C/100 h). |
4 Conclusions
The tensile properties of large cast H282 has been improved by optimizing heat treatment to eliminate cracks. Detailed microstructure characterizations and calculations were conducted to reveal the formation of cracks during previous heat treatment and the following conclusions were drawn:
Grain boundary cracks and grain boundary Ti, Mo-rich MC carbides cracks were found in the tensile sample underwent HT1, these cracks are believed to decrease the tensile strength of the large cast H282.
Microstructural results and thermodynamic calculations showed that the grain boundary cracking was most likely to be the dissolution of matrix and μ phase. By 3D reconstruction and interface residual stress calculation, grain boundary MC cracking is caused by too high cooling rate after homogenization at 1200 °C.
The interface stress calculation shows that the rate of temperature change has a dominant effect on the thermal misfit stress, while the aspect ratio of the carbides between 1 and 5 are sensitive to the thermal misfit stress. Lowering cooling rate can eliminate grain boundary MC cracking, but this will increase the size of γ/ particles which will reduce the creep properties.
The modified HT2 treatment works for the large cast H282, results in tensile strength of 671 MPa for the thin section and 554 MPa for the thick section. However, the tensile properties of the thick section are much lower than that of the thin section, because of some localized melting, suggesting that chemical composition optimization of the cast H282 requires further work.
Funding
This research was sponsored by Nanjing Vocational University of Industrial Technology Introduce Talent Research Start-up Funding [YK20-04-04]. The materials and equipment used in this research were supported by Goodwin Steel Casting LTD, UK and Loughborough University, UK.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors have no conflict to disclose.
Data availability statement
All data has already been reported in the manuscript.
References
- S. Park, J. Kim, M. Yoon, D. Rhim, C. Yeom, Thermodynamic and economic investigation of coal-fired power plant combined with various supercritical CO2 Brayton power cycle, Appl. Thermal Eng. 130, 611–623 (2018) [CrossRef] [Google Scholar]
- T. Dudziak, K. Jura, A. Polkowska, V. Deodeshmukh, M. Warmuzek, M. Witkowska, K. Chruściel, Steam oxidation resistance of advanced steels and Ni-based alloys at 700° C for 1000 h, Oxidat. Metals 89, 755–779 (2018) [CrossRef] [Google Scholar]
- L.M. Pike, Development of a fabricable gamma-prime (γ′) strengthened superalloy, Superalloys 2008, 191–200 (2008) [Google Scholar]
- Y.J. Kim, J.H. Park, Y.S. Ahn, Comparison of creep properties of cast and wrought Haynes 282 superalloy, Adv. Mater. Sci. Eng. 2018 (2018) [Google Scholar]
- H. Matysiak, M. Zagorska, J. Andersson, A. Balkowiec, R. Cygan, M. Rasinski, K.J. Kurzydlowski, Microstructure of Haynes® 282® superalloy after vacuum induction melting and investment casting of thin-walled components, Materials 6, 5016–5037 (2013) [CrossRef] [PubMed] [Google Scholar]
- P.D. Jablonski, J.A. Hawk, C.J. Cowen et al., Processing of advanced cast alloys for A-USC steam turbine applications, JOM 64, 271–279 (2012) [CrossRef] [Google Scholar]
- Y.J. Kim, J.H. Park, Y.S. Ahn, Comparison of creep properties of cast and wrought Haynes 282 superalloy, Adv. Mater. Sci. Eng. (2018). doi: 10.1155/2018/2048959 [Google Scholar]
- S. Briks, S. Roberts, R. Leese, Advances in materials technology for fossil power plants, in Adv. Mater. Technol. Foss. Power Plants Proc. 7th Int. Conference (2013) pp. 491–503 [Google Scholar]
- M. Dhondt, I. Aubert, N. Saintier, J.M. Olive, Characterization of intergranular stress corrosion cracking behavior of a FSW Al-Cu-Li 2050 nugget, Mech. Ind. 16, 401 (2015) [CrossRef] [EDP Sciences] [Google Scholar]
- J. Carrier, E. Markiewicz, G. Haugou, D. Lebaillif, N. Leconte, H. Naceur, Thermal effect of the welding process on the dynamic behavior of the HSS constitutive materials of a fillet welded joint, Mech. Ind. 18, 301 (2017) [CrossRef] [EDP Sciences] [Google Scholar]
- K.A. Unocic, M.M. Kirka, E. Cakmak, D. Greeley, A.O. Okello, S. Dryepondt, Evaluation of additive electron beam melting of Haynes 282 alloy, Mater. Sci. Eng. A 772, 138607 (2020) [CrossRef] [Google Scholar]
- A. Deshpande, S. Deb Nath, S. Atre, K. Hsu, Effect of post processing heat treatment routes on microstructure and mechanical property evolution of Haynes 282 Ni-based superalloy fabricated with selective laser melting (SLM), Metals 10, 629 (2020) [CrossRef] [Google Scholar]
- A. Ramakrishnan, G.P. Dinda, Microstructure and mechanical properties of direct laser metal deposited Haynes 282 superalloy, Mater. Sci. Eng. A 748, 347–356 (2019) [CrossRef] [Google Scholar]
- J. Boswell, J. Jones, N. Barnard, D. Clark, M. Whittaker, R. Lancaster, The effects of energy density and heat treatment on the microstructure and mechanical properties of laser additive manufactured Haynes 282, Mater. Des. 2021, 109725 (2021) [CrossRef] [Google Scholar]
- L.M. Pike, Development of a fabricable gamma-prime (γ′) strengthened superalloy, Superalloys 2008, 191–200 (2008) [Google Scholar]
- L.O. Osoba, O.A. Ojo, Influence of laser welding heat input on HAZ cracking in newly developed Haynes 282 superalloy, Mater. Sci. Technol. 28, 431–436 (2012) [CrossRef] [Google Scholar]
- Y. Yang, R.C. Thomson, R.M. Leese, S. Roberts, Microstructural evolution in cast Haynes 282 for application in advanced power plants, in D. Gandy, J. Shingledecker (eds.), Advances in Materials Technology for Fossil Power Plants, Proceedings of the 7th International Conference (EPRI 2013), Waikoloa, Hawaii, USA. ASM International (2013). pp. 143–154 [Google Scholar]
- Y. Yang, Microstructural evolution of large cast Haynes 282 at elevated temperature, Crystals 11, 867 (2021) [CrossRef] [Google Scholar]
- S. Singh, J. Andersson, Heat-affected-zone liquation cracking in welded cast Haynes 282, Metals 10, 29 (2019) [CrossRef] [MathSciNet] [Google Scholar]
- P.D. Jablonski, C.J. Cowen, Homogenizing a nickel-based superalloy: thermodynamic and kinetic simulation and experimental results, Metall. Mater. Trans. B 40, 182–186 (2009) [CrossRef] [Google Scholar]
- R. Davies, A.T. Dinsdale, J. Gisby, J.A. Robinson, S. Martin, MTDATA − thermodynamic and phase equilibrium software from the National Physical Laboratory. Calphad-computer Coupling of Phase Diagrams and Thermochemistry, 26, 229–271 (2002) [CrossRef] [Google Scholar]
- N. Saunders,. Phase Diagram Calculations for Ni-based superalloys. Superalloys, 101–110 (1996) [Google Scholar]
- G. Evans, Metal/Ceramic Interfaces: Relationships Between Structures, Chemistry and Interfaces (University of California, 1991) [Google Scholar]
- T. Clyne, P. Withers, An introduction to metal matrix composites: The Eshelby approach to modelling composites (Cambridge University Press, 1993) [CrossRef] [Google Scholar]
- A. Weimer, Carbide, Nitride and Boride Materials Synthesis and Processing, First Edit (Chapman & Hall, 1997) [Google Scholar]
- B.H.L. Logan, Effect of the quenching rate on susceptibility to intercrystalline corrosion of heat treated 24s aluminium alloy sheet, J. Res. Natl. Bur. Stand. 26, 321–329 (1934) [Google Scholar]
- K.Y. Shin, J.H. Kim, M. Terner, B.O. Kong, H.U. Hong, Effects of heat treatment on the microstructure evolution and the high-temperature tensile properties of Haynes 282 superalloy, Mater. Sci. Eng.: A 751, 311–322 (2019) [CrossRef] [Google Scholar]
Cite this article as: Y. Yang, Optimization of large cast Haynes 282 based on thermal induced cracks: formation and elimination, Mechanics & Industry 25, 12 (2024)
All Tables
Quantified compositions of different Mo rich grain boundary phases in wt.% in as cast condition.
Tensile properties of thin and thick section of H282 after modified heat treatment.
All Figures
 |
Fig. 1 A picture of a cast step block (thin section, thick section) of the cast H282 materials; red dash lines are cutting lines. |
| In the text | |
 |
Fig. 2 Micrographs of H282 after the HT1(tensile sample): grain boundary cracks and grain boundary precipitate cracks. |
| In the text | |
 |
Fig. 3 The effect of the HT1 on grain boundary blocky MC: (a) after the HT1-1 treatment; (b) after the HT1-2 treatment; (c) after the HT1-3 treatment and (d) after the HT1-4 treatment. |
| In the text | |
 |
Fig. 4 (a) Predicted equilibrium phase diagram using the M6C composition and (b) Predicted equilibrium phase diagram using the μ phase composition. |
| In the text | |
 |
Fig. 5 (a) A schematic diagram of the matrix-carbide-matrix system and (b) CTE plot comparing the measured and published values of H282 [3]. |
| In the text | |
 |
Fig. 6 A 3-D reconstruction of grain boundary blocky MC with cracks. |
| In the text | |
 |
Fig. 7 A plot of residual stress against aspect ratio as a function of different rates of change in temperature. |
| In the text | |
 |
Fig. 8 Micrographs showing microstructure after the HT with furnace cooling: (a, b) samples underwent HT1-2(F); (c) samples underwent HT1(F). |
| In the text | |
 |
Fig. 9 Micrographs showing microstructure after the HT2: (a, b) the thin section; (c, d) the thick section. |
| In the text | |
 |
Fig. 10 A micrograph of thick section after HT3 (homogenized at 1100 °C/100 h). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.